
New Progress in the Research of Glass Corrosion Mechanism
Recently, the Advanced Laser and Optoelectronic Functional Materials Department of the Shanghai Institute of Optics and Fine Mechanics has made has made new progress in the research of glass corrosion mechanism. They proposed a new mechanism for the formation of gel layer in the corrosion layer, which perfectly explains the key issues that have not been agreed in the research of glass corrosion mechanism for a long time in the academic community. The relevant research results were published in Acta Materialia under the title "Exploring the Corrosion Mechanism of Oxide Glasses Using Advanced Solid-State Nuclear Magnetic Resonance Spectroscopy".
The chemical stability of glass is crucial for its applications in fields such as biology, optics, and nuclear waste solidification. A deep understanding of the corrosion mechanism of glass is a prerequisite for achieving its chemical stability control. Although research on the corrosion mechanism of glass has been conducted for over 70 years, no consensus has been reached. The focus of controversy is on the formation mechanism of the gel layer in the glass corrosion layer, especially on the cause of the sudden change of chemical concentration near the interface between the gel layer and the glass phase. The previous main viewpoints unanimously believed that this sudden change in chemical concentration was caused by the dissolution of glass components and their subsequent deposition in the glass, although these viewpoints have different explanations for the sedimentation mechanism. However, none of these views can explain why gel is still formed when the solution has not reached the saturation state.
In this study, the researchers comprehensively analyzed the chemical changes and atomic scale structural changes in the process of glass corrosion through a variety of advanced solid-state nuclear magnetic resonance technologies, and revealed that the glass network was largely damaged by water during the formation of the gel layer, while in the hydrated glass layer, water was mainly filled in the gaps of the glass network, with few broken glass network structures. The researchers believe that it is this remarkable difference in structure that causes the sudden change of chemical concentration near the interface between the gel layer and the glass phase. This new discovery will advance the understanding of the corrosion mechanism of glass.
This work was supported by the China National Project titled, “Development and Verification of Two-Step Cold Crucible Vitrification Engineering-Scale Prototype” (grant no. FKY1683ZHG001SSJS-B01-001)
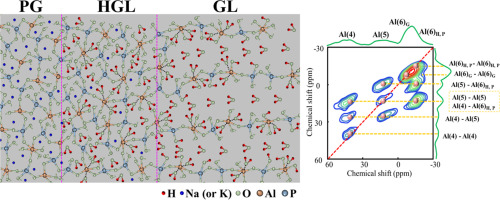
Left: The structure evolution of phosphate glass during the immersion process. Right: comparison between the 27Al MAS spectrum (top) and the 27Al{1H}1D
HMQC spectrum (bottom) of the corroded glass after 7days of immersion.
Article website: https://doi.org/10.1016/j.actamat.2024.120164