
The Change Mechanism of Eu2+ Emission Wavelength in Barium Borate Glass
Alkali metal borate glass has high transparency and good rare earth solubility, and is often used as a luminescent matrix glass for rare earth ions. Eu2+ is a common rare earth luminescent ion that is commonly doped as an activator in matrix materials such as glass and phosphor. The 5d energy level of the outer layer of Eu2+ is susceptible to the influence of the external environment, resulting in the energy level splitting, resulting in the shift of the luminescence wavelength of the 5d-4f transition. Therefore, Eu2+ emits a variety of light ranging from violet to red in different according to the substrate materials, which makes Eu2+ doped materials very versatile.
The electron paramagnetic resonance spectroscopy (EPR) of Eu2+ is characterized by a "U" shaped spectrum, so EPR can be used to detect the presence of Eu2+ as well as the coordination field environment around Eu2+. In this work, the effect of glass components on the coordination field strength around Eu2+ was analyzed by studying the changes of Eu2+ characteristic EPR signal peaks in barium borate glass samples.
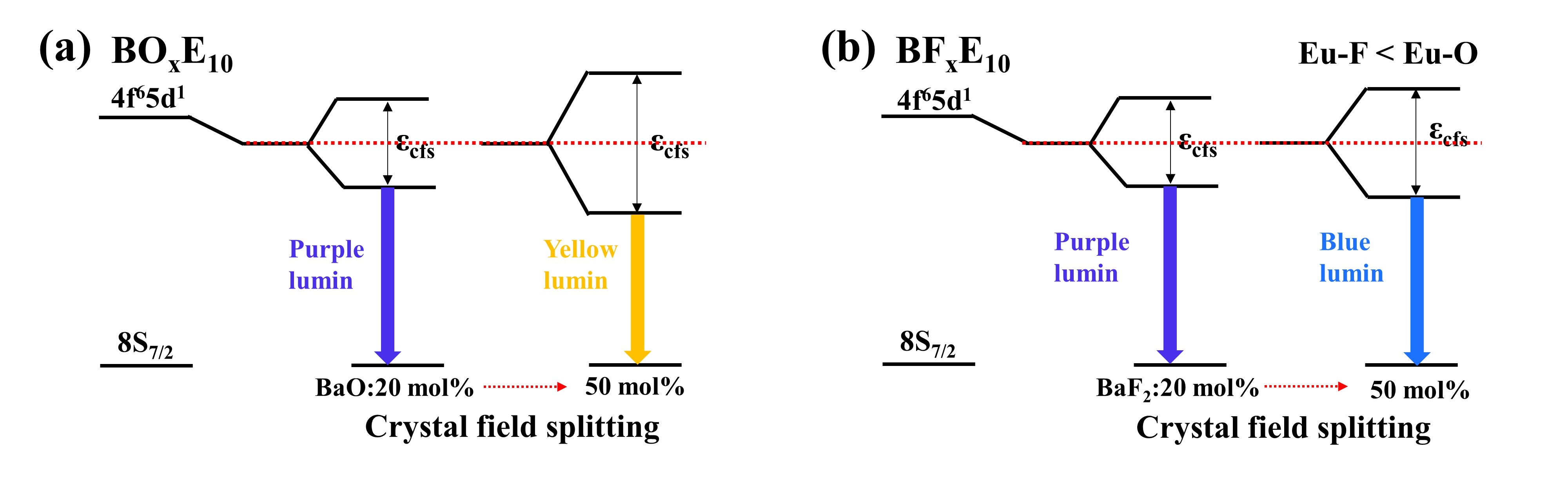
Fig.1 Schematic diagram of the emission red-shifts in (a) the BOxE10 and (b) BFxE10 glasses.
In this study, Eu2+-doped barium borate glass was prepared under CO atmosphere, and the mechanism of Eu2+ fluorescence spectrum change was studied. The results show that the increase of barium content in glass leads to the enhancement of the coordination field intensity around Eu2+ ions, and the degree of 5d energy level splitting of Eu2+ increases, resulting in the red shift of the emission spectrum. However, the introduction of fluorine in the glass will cause the weakening of the coordination field intensity around Eu2+ ions, which will inhibit the redshift of the emission spectrum. At the same time, it was found that the increase of Eu2+ concentration also caused the redshift of the emission spectrum, which was attributed to the nephelauxetic effect of the 5d level, which led to the downward shift of the center of the 5d level. In this study, we systematically and scientifically explained the two mechanisms that cause the change of Eu2+ luminescence wavelength, and found that fluoride ions can provide a low-field strength neighbor coordination environment for the luminescence center, which can guide the adjustment of the luminescence color of Eu2+ doped glass, phosphor and other materials.
The research was supported by the National Natural Science Foundation of China.
Article website: https://doi.org/10.1016/j.ceramint.2023.10.285